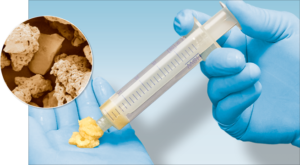
Biologics
MRS Ortho proudly distributes the full line of Xtant Medical, Extremity Medical, ConMed & Medline surgical systems.


Each surgical system listed below links to the Xtant website, where you can find more information on that specific system.
Xtant’s 3Demin Technology utilizes demineralized cortical bone fibers that are entangled and shaped into sizes engineered to complement specific surgical applications. This unique process creates an interconnected graft material that contains BMPs and other growth factors necessary for the promotion of new bone formation. 3Demin allografts are flexible upon hydration and each allograft has a sterility assurance level of 10-6 via low-dose gamma sterilization. 3Demin allografts are also available as loose cortical fibers in three volume options.
• Derived from 100% human bone – maximum allograft content; no extraneous carriers that may impede bone healing
• Osteoinductive potential – validated for positive osteoinductive capacity
• Osteoconductive – DBM provides an ideal scaffold that directs and supports bone formation
• Excellent handling characteristics – retains shape and resists irrigation and migration
• Convenient and ready to use – stores at room temperature and ready for immediate use
• Sterility assurance level (SAL) 10-6 – terminal sterilization ensures the highest level of patient safety
OsteoSelect is a superior bone graft that maintains its placement during surgery without sticking to gloves. It has been tested and consistently shown to have an osteoinductive response, proving the expertise of Xtant Medical in processing demineralized bone matrix (DBM). OsteoSelect PLUS combines demineralized cortical chips with OsteoSelect DBM putty to eliminate the need for intra-operative mixing, ensuring optimal osteoinductivity. It can be used as a standalone bone graft in spinal procedures and has been proven to be equivalent to iliac crest autograft in preclinical spinal fusion models. Patient safety is ensured through terminal sterilization and viral inactivation.
OsteoSponge is a patented form of demineralized bone matrix (DBM) made entirely from cancellous bone. It provides a natural scaffold for cellular growth and contains bone-growth-inducing proteins to promote healing. OsteoSponge is malleable and can fill and conform to irregular bony defects, expanding to completely fill a void after placement. It is an ideal bone graft for use in spinal fusion devices, arthrodesis, or fracture sites. OsteoSponge is composed of 100% human demineralized cancellous bone, has interconnected porosity, and has osteoconductive and osteoinductive potential. It is compressible, radiolucent for accurate follow-up, sterile, has a 5-year shelf life, and can be stored at room temperature.
OsteoWrap is a bone graft made from 100% human demineralized cortical bone that is flexible while maintaining allograft integrity. It can be easily sized with scissors or a scalpel and sutured in place, making it versatile and ideal for use in a variety of surgical procedures, including spinal, neurological, cranio-maxillofacial, reconstructive, and general orthopedic surgeries. OsteoWrap is osteoconductive and osteoinductive and has flexible handling characteristics that enable it to contour to patient anatomy. It is radiolucent for accurate follow-up assessment of bone formation and is sterile.
OsteoFactor is a uniquely processed allograft that contains retained growth factors found within the endosteum layer of allograft bone. Unlike the various growth factor-based products on the market today, OsteoFactor is not limited to a single growth factor but contains a wide array of naturally occurring proteins and peptides that support bone formation and remodeling.
• Wide array of growth factors
• Elevated level of natural BMP-2
• High growth factor binding and long-term sustained release
• Easy to use and customizable
• Safe and non-immunogenic
The SimpliMix™ Graft Delivery Device is designed for targeted delivery of hydrated allograft, autograft, or synthetic bone graft materials to an orthopedic surgical site, while maximizing material utilization
• Integrated Stylet
• Vented Configuration
• Tapered Cap and Wide Bore Cannula
• Easy to use
Matriform Si was developed to resemble the composition and porous structure of natural human bone. Comprised of 96% pure phase β-TCP granules, 4% silicate and collagen, Matriform Si provides the ideal biomimetic scaffold for spinal fusion procedures. The flexible strip offers excellent handling and shape memory ensuring direct contact with the surface of healthy bone.
• 99% pure phase β-TCP
• Homogenous incorporation of silicate
• Total porosity ≥ 75%
• Multiporous, osteoconductive matrix
• Fluid retention
• Room temperature storage
Traditional product offerings include Ilium Tricortical Blocks, Crushed Cancellous Chips, Unicortical Blocks, Fibula Segments, and Femoral Struts. Features of these products are listed below:
• Sterile R
• 5 year shelf life
• Room-temperature storage
• Osteogenic Potential- Proprietary processing preserves adult mesenchymal stem cells while removing red and white blood cells.
• Osteoinductive Potential- Cortical fiber demineralized bone matrix provides a high-quality osteoinductive signal. Additional growth factors are captured from bone lining cells and restored back into the product.*
• Osteoconductive- The cortical fiber demineralized bone matrix and cancellous bone create a robust scaffold.
• Ease of Use- 10-minute thaw time in a room-temperature water bath. OsteoVive Plus is DMSO-free and immediately implantable upon thawing in a ready-to-use syringe.
Sports Medicine allografts are an excellent replacement option for deficient or torn cruciate ligament reconstructions and revisions. Benefits include consistent, superior quality and precise measurements. As with all of Xtant Medical’s allografts, safety and efficacy is an absolute priority. All tendons are distributed sterile via low-dose gamma irradiation.
• Sterile R
• Stored and distributed as a frozen product
• 5 year shelf life
Contact your local Xtant sales representative
To learn more about Xtant, visit their website.
For Xtant news and updates, visit their website
To contact Xtant, visit their website


Each surgical system listed below links to the Extremity Medical website, where you can find more information on that specific system.
Omni Arch® is a medical device designed to treat flatfoot deformity. This lightweight implant is made of a titanium alloy and is anatomically contoured to fit the unique shape of each patient’s foot. The device is inserted through a minimally invasive surgical procedure and provides immediate relief by supporting the arch and stabilizing the ankle joint. The Omni Arch is also adjustable, allowing the surgeon to fine-tune the level of support during the surgery.
Contact your local Extremity Medical sales representative
To learn more about Extremity Medical, visit their website.
For Extremity Medical press releases & articles visit their website
To contact Extremity Medical, visit their website


Each surgical system listed below links to the ConMed website, where you can find more information on that specific system.
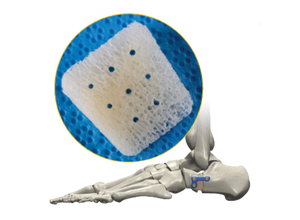
AlloAid® DIP
ENGINEERED STERILE ALLOGRAFT FOR ARTHRODESIS
The AlloAid® DIP is a sterile, engineered Allograft. The Allograft is supplied in 2 sizes. The AlloAid® DIP complies with all FDA, AATB and state regulatory requirements for donor screening, recovery and testing.
- Designed with Positioning Ramps to help accurately position the implant and ensure a press-fit.
- Sterile Allograft has conductive properties.
- No post-op hardware removal needed
- Available in 2 sizes.
- This allograft complies with all FDA, AATB and State Regulatory requirements for donor screening, recovery and testing.
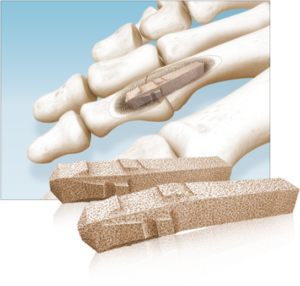
Engineered with multiple wedge facets designed to anchor the BioNail in the bone. AlloAid BioNail can be used for fixation and augmentation of small bone osteotomies of the foot.
- Sterile Allograft, 10-6 SAL
- Engineered 100% Allograft
- No hardware concerns
- 2 Diameters & 3 Lengths
The Allograft is supplied in 2 sizes featuring both straight and 10° angled configurations. The AlloAid® PIP complies with all FDA, AATB and state regulatory requirements for donor screening, recovery and testing.
- Designed with Positioning Ramps to help accurately position the implant and ensure a press-fit.
- Sterile aAllograft has conductive properties.
- No post-op hardware removal needed.
- Available in both straight & 10° angled configurations.
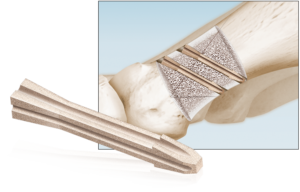
AlloAid® Wedges
Cotton and Evans Wedge Implants
- Dense Cancellous Porous Structure for Optimal Bone Incorporation
- Packaged in Saline for Ideal Structural Support
- Sterile and Ready for Use
AlloAid Crunch, DBM Putty
- Available in flowable, formable putty and crunch in syringe
- Donor recovery and screening performed according to AATB and FDA guidelines
- Crunch consists of DBM putty & cortical cancellous bone chips
100% Cancellous Bone Sponge Cubes
- Moldable, sterile, demineralized/compressive bone matrix
- Cut and molded as needed; sponge-like fit and fill capabilities
- Natural porous scaffold encourages osteoconductivity, promotes vascularization and stimulates new bone growth
- Add bone marrow aspirate or platelet-rich plasma to create a composite graft
AlloAid® Dermal Matrix is made using sterilized human acellular dermis (ACD), processed to maintain the integrity of the biologic and structural components of the extracellular matrix. This ensures that the product minimizes inflammation, encourages vascularization and cell repopulation. AlloAid® is gamma irradiated to SAL 10-6, ensuring that it is completely sterile and free from any potential contaminants. The epidermal layer has been removed, eliminating the need to provide side-specific placement. AlloAid® remodels naturally without the need for chemical crosslinking, preserves remodelability, and can be stored at ambient temperatures
BioV® Bioactive Matrix is a state-of-the-art bone graft substitute that combines bioactive glass and demineralized bone matrix to facilitate new bone formation. Bioactive Glass is osteoconductive and osteopromotive, while Demineralized allograft bone is osteoinductive, and In2Bones’ patented process integrates these elements for optimal results. BioV® is easily prepared, moldable, extrudable, and resistant to migration, making it perfect for surgical handling, graft stability, and osteoproductivity in bone grafting applications.
i2b® Resorbable Bead Kit / Rapid Set
The i2b® Resorbable Bead Kit is designed to create easy-to-use bio-resorbable beads for filling osseous defects. i2b® Resorbable Bead Kits allow surgeons to cast 100% pure synthetic resorbable bone void filler Beads in two sizes: 3.5 and 4.5mm. The beads provide a safe alternative to human or animal cadaver bone that completely eliminates the potential for disease transmission. The Mat features vented pockets to minimize air pockets and maximize the fill rate.
Each sterile i2b® Resorbable Bead Kit includes:
- Bone Void Filler Powder
- Sterile Water
- Mixing Spatula
- Bead Mat
Tribio™ Backfill Plugs
UNIQUE BONE VOID FILLER
Contact your local ConMed sales representative
To learn more about ConMed, visit their website.
For ConMed press releases & articles, visit their website
To contact ConMed, visit their website


Each surgical system listed below links to the Medline website, where you can find more information on that specific system.
ACTIGLASS™ Synthetic Bioactive Putty
Small (3.75g) and Medium (7.5g)
ACTISTIM™ Demineralized Fiber Putty
1cc, 2.5cc, 5cc, and 10cc
ACTIVI ™️ Fiber Viable Bone Matric
1cc, 2cc, 5cc, and 10cc

1x1cm, 2x2cm, 2x3cm, 4x4cm, 4x6cm, 4x8cm
1-4mm: 5cc, 10cc, 15cc, and 30cc
4-10mm: 10cc, 15cc, and 30cc

1cc, 2.5cc, 5cc, and 10cc
Pre-Hydrated Evans & Cotton Wedges
Dense cancellous Evans and Cotton Wedges are pre-shaped and pre-hydrated to eliminate idle OR time associated with cutting and soaking a traditional bone wedge. The wedges are sterilized in a solution rather than freeze-dried, to preserve the structural integrity of the graft and avoid intra- and post-operative crumbling. The system comes equipped with trials to determine the proper wedge size.
Sizes/Options:
Evans: 6mm, 8mm, 10mm, and 12mm
Cotton: 5mm, 6mm, and 7mm

Pre-Hydrated MTP Revision Bioimplants
Dense cancellous MTP Revision Bioimplants are pre-hydrated in saline to eliminate idle OR time associated with cutting and soaking a traditional bone graft. The bioimplants are sterilized in saline rather than freeze-dried, to preserved the structural integrity of the graft and to avoid intraoperative graft fracture when reaming. Two distinct size options are available for unique revision scenarios: a small option designed to backfill metatarsal head voids and a large option design to bridge a larger gap and restore length. The bioimplants feature a flat-cut, cannulated designed to be used with a provided 1.6mm guidewire, MTP cup and cone reamers, and acorn reamer. Reamers are used for intraoperative shaping and graft site preparation, which may vary depending on revision scenario and surgeon-preference or technique.
Sizes/Options:
Small: Ø11 x 15mm
Large: Ø18 x 15mm
Contact your local Medline sales representative
To learn more about Medline, visit their website.